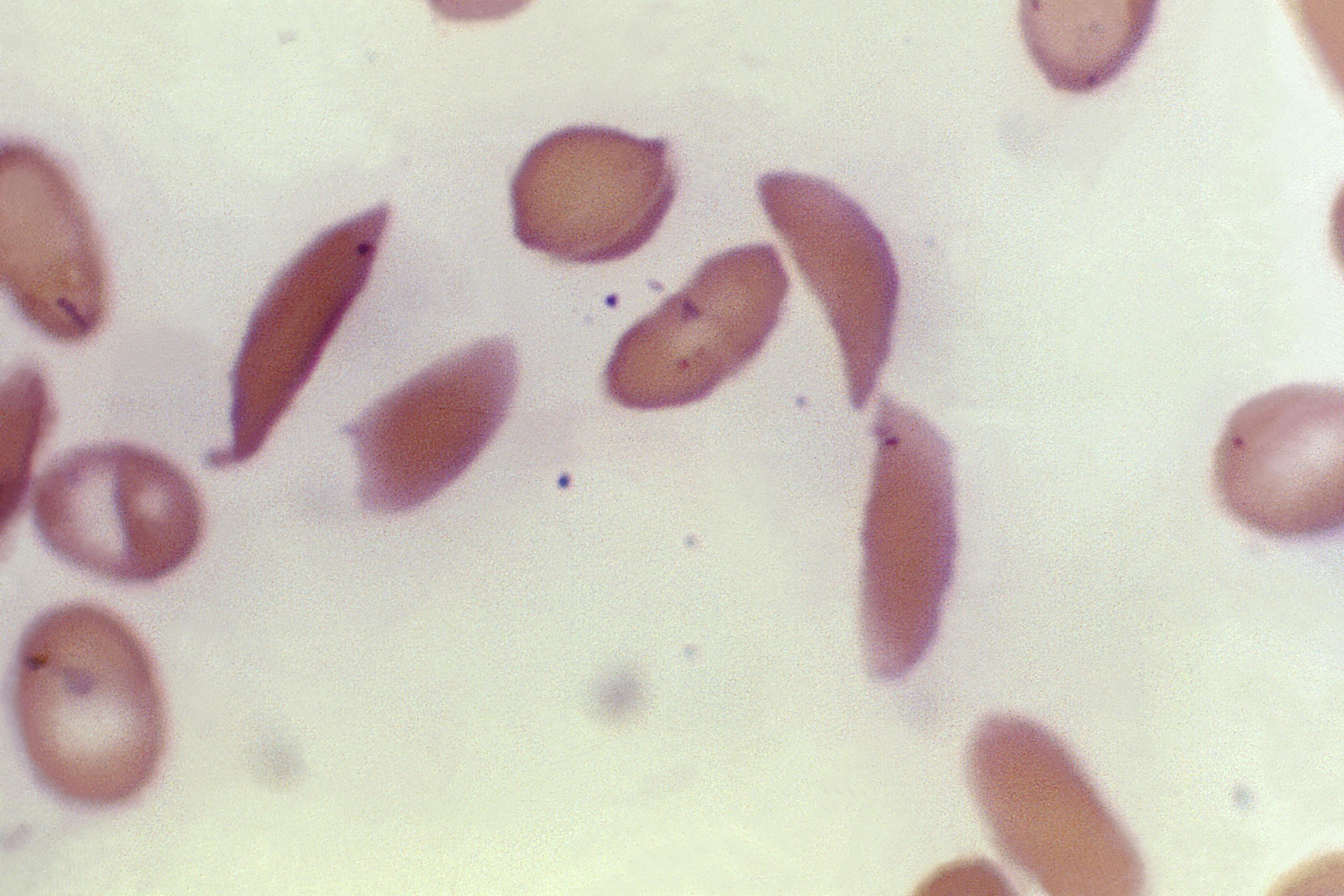
Sickle cell disease, which occurs in one of every 365 Black births, is caused by a gene mutation that alters the shape of blood cells. These cells get stuck in blood vessels throughout the body, causing organ damage and excruciating pain.
“There's damage being done to the heart, to the brain, to the lungs, to the kidneys, to the liver. … We think of it as a blood disorder, but it affects the entire body,” said Dr. Melissa McNaull, a physician at the University of Mississippi Medical Center.
McNaull specializes in children's cancer and blood disorders and trains family practitioners in the Delta on how to care for sickle cell patients.
Previously, the only way to treat sickle cell was through bone marrow transplants from a healthy sibling or parent. The new exa-cel gene therapy treatment involves removing and editing bone marrow stem cells. When the modified cells are replaced, they allow the production of fetal hemoglobin that functionally cures the disease.
“[The patients] have no anemia,” McNaull said. “They're not breaking down their cells fast. They're not having pain. We don't have the long enough data, but we extrapolate that they're not having further organ damage. So they're healthy, and they feel great.”
One problem for patient access is the cost. This new treatment is expected to cost millions of dollars per patient, though official numbers have not been released. But McNaull thinks insurers will eventually cover exa-cell, like they do with bone marrow transplants, due to the high cost of lifelong care for patients with the disease.
“Bone marrow transplants used to be somewhat rare,” McNaull said. “We went through the same thing with that. We said, ‘oh, that's so much money insurance is gonna balk,’ and at first insurance did balk. Now, insurance doesn't bat an eye… I think this is going to be along those lines.”
Full FDA approval for exa-cel could come as soon as December 8.